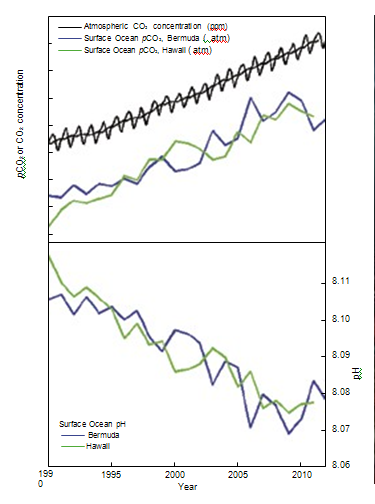
figure 7. As CO2 in the air has
increased, there has been an
increase in the CO2 content of the
surface ocean (upper box), and a
decrease in the seawater pH (lower
box). Source: adapted from Dore et al.
(2009) and Bates et al. (2012). | Direct observations of ocean chemistry have shown that the chemical balance of seawater
has shifted to a more acidic state (lower pH) [Figure 7]. Some marine organisms (such
as corals and some shellfish) have shells composed of calcium carbonate which dissolves
more readily in acid. As the acidity of sea water increases, it becomes more difficult for
them to form or maintain their shells. CO2 dissolves in water to form a weak acid, and the oceans have absorbed about a third of the CO2 resulting
from human activities, leading to a steady decrease in ocean pH levels. With increasing atmospheric CO2,
the chemical balance will change even more during the next century. Laboratory and other experiments
show that under high CO2 and in more acidic waters, some marine species have misshapen shells and
lower growth rates, although the effect varies among species. Acidification also alters the cycling of
nutrients and many other elements and compounds in the ocean, and it is likely to shift the competitive
advantage among species, with as-yet-to-be-determined impacts on marine ecosystems and the food web. |